Exploring NeuroStar TMS: Your Questions Answered
Discover the benefits of NeuroStar TMS therapy, a non-invasive, FDA-cleared treatment for depression.
Common Patient Question
NeuroStar Highlights
- Non-drug, non-invasive
- No systemic side effects
- Covered by most insurances
- FDA-cleared technology
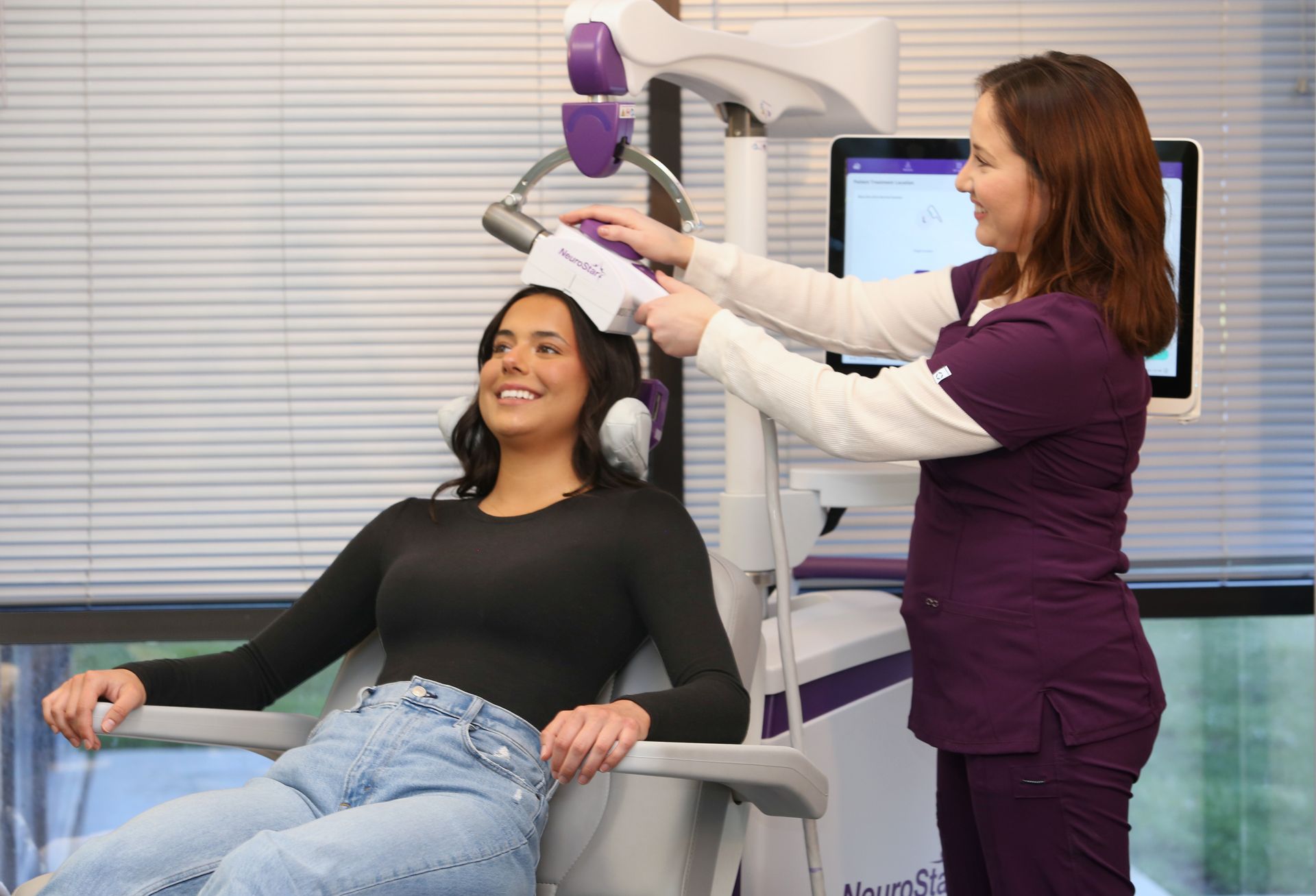
What is NeuroStar?
NeuroStar is a safe, effective, non-drug depression treatment that uses focused magnetic pulses, similar in strength to an MRI, to revitalize connections in an area of the brain involved in regulating your mood. When these connections are reawakened, many people experience measurable relief from their depression.
Does it work?
In a study of "real-world" outcomes (meaning actual NeuroStar patients), 83% of people who completed the full NeuroStar treatment cycle experienced a measurable decrease in the severity of their depression, and 62% of those completing treatment saw full remission - meaning their depression effectively "went away."
Does it hurt?
While there may be some minor discomfort at the treatment site (where the device touches your head), it generally subsides within the first week or treatment. There is no sedation, or impact on your alertness. You can read, watch TV, or talk with your treatment coordinator during your session, and you can drive home immediately after treatment.
Does insurance cover it?
Over 300 million people have insurance plans that cover NeuroStar, including Medicare and Tricare. Conditions and coverage can vary by insurance company, and your NeuroStar Team Lead will help you determine your benefits and coverage. We also offer cash-pay and patient financing options if desired, talk with your NeuroStar representative for more details.
Who is NeuroStar for?
NeuroStar is FDA-cleared for adults with Major Depressive Disorder who have not found relief from antidepressant medications. A NeuroStar leader will walk you through the full qualifications for treatment during your initial consultation.
[1] Sackeim HA, et al. (2020) J. Affect. Disord. 277:65-74. Based on a real-world, retrospective study using CGI-S and a sample size of 615 patients.
Click the button below
to complete the questionnaire and find out if you qualify for
TMS treatment.
Begin your journey to improved mental health today!